Tstk flows into. Tricarboxylic acid cycle (TCA cycle)
- General idea. Characteristics of the stages of the cycle cycle.
- Final products of the TFC.
- Biological role of the TCA cycle.
- Regulation of the TCA cycle.
- Disturbances in the operation of the central heating system.
· GENERAL VIEW. CHARACTERISTICS OF CTC STAGES
The tricarboxylic acid cycle (TCA cycle) is main, cyclic, metabolic pathway, in which the oxidation of active acetic acid and some other compounds formed during the breakdown of carbohydrates, lipids, proteins occurs and which provides the respiratory chain with reduced coenzymes.
CTK was opened in 1937 G. Krebs. He summarized the experimental studies available at that time and constructed a complete diagram of the process.
TCA cycle reactions proceed in mitochondria under aerobic conditions.
At the beginning of the cycle (Fig. 6), active acetic acid (acetyl-CoA) condenses with oxaloacetic acid (oxaloacetate) to form citric acid (citrate). This reaction is catalyzed citrate synthase .
The citrate is then isomerized to isocitrate. Isomerization of citrate is carried out by dehydration to form cis-aconitate and its subsequent hydration. Catalysis of both reactions provides aconitase .
At the 4th stage of the cycle, oxidative decarboxylation of isocitrate occurs under the influence of isocitrate dehydrogenase (ICDG) with education a-ketoglutaric acid, NADH(H +) or NADPH(H +) and CO 2 . NAD-dependent IDH is localized in mitochondria, and NADP-dependent enzyme is present in mitochondria and cytoplasm.
During the 5th stage, oxidative decarboxylation of a-ketoglutarate occurs with the formation active succinic acid (succinyl-CoA), NADH(H) and CO2. This process is catalyzed a-ketoglutarate dehydrogenase complex , consisting of three enzymes and five coenzymes. Enzymes: 1) a-ketoglutarate dehydrogenase associated with the coenzyme TPP; 2) transsuccinylase, the coenzyme of which is lipoic acid;
3) dihydrolipoyl dehydrogenase associated with FAD. In the work of a-ketoglutarate dehydrogenases
This complex also involves coenzymes CoA-SH and NAD.
At the 6th stage, the high-energy thioester bond of succinyl-CoA is cleaved, coupled with the phosphorylation of GDP. Are formed succinic acid (succinate) And GTP (at the level of substrate phosphorylation). The reaction is catalyzed succinyl-CoA synthetase (succinylthiokinase) . The phosphoryl group of GTP can be transferred to ADP: GTP + ADP ® GDP + ATP. The reaction is catalyzed with the participation of the enzyme nucleoside diphosphokinase.
During the 7th stage, succinate is oxidized under the influence of succinate dehydrogenase with education fumarateand FADN 2.
At the 8th stage fumarate hydratase ensures the addition of water to fumaric acid to form L-malic acid (L-malate).
L-malate at the 9th stage under the influence malate dehydrogenase oxidizes to oxaloacetate, the reaction also produces NADH(H+). The metabolic pathway closes on oxaloacetate and again repeats itself, purchasing cyclical character.
Rice. 6. Scheme of reactions of the tricarboxylic acid cycle.
· FINAL PRODUCTS
The overall CTC equation has the following form:
// ABOUT
CH 3 – C~ S-CoA + 3 NAD + + FAD + ADP + H 3 PO 4 + 3 H 2 O ®
® 2 CO 2 + 3 NADH(H +) + FADH 2 + ATP + CoA-SH
Thus, the final products of the cycle (per 1 turnover) are reduced coenzymes - 3 NADH (H +) and 1 FADH 2, 2 molecules of carbon dioxide, 1 molecule of ATP and 1 molecule of CoA - SH.
· BIOLOGICAL ROLE OF THE TCA cycle
The Krebs cycle performs integration, amphibolic (i.e. catabolic and anabolic), energy and hydrogen donor role.
Integration role is that the TTC is final common oxidation pathway fuel molecules - carbohydrates, fatty acids and amino acids.
Happens at the TsTK oxidation of acetyl-CoA iscatabolicrole.
Anabolic the role of the cycle is that it supplies intermediate products For biosynthetic processes. For example, oxaloacetate is used to synthesize aspartate, a-ketoglutarate – for education glutamate, succinyl-CoA – for synthesis heme.
One molecule ATP is formed in the TCA at the level substrate phosphorylation is energy role.
Hydrogen donor The role is that the TCA cycle provides reduced coenzymes NADH(H+) and FADH 2 the respiratory chain, in which the oxidation of hydrogen from these coenzymes to water occurs, coupled with the synthesis of ATP. When one molecule of acetyl-CoA is oxidized in the TCA cycle, 3 NADH(H +) and 1 FADH are formed 2
The ATP yield during the oxidation of acetyl-CoA is 12 ATP molecules (1 ATP in the TCA cycle at the level of substrate phosphorylation and 11 ATP molecules during the oxidation of 3 molecules of NADH(H +) and 1 molecule of FADH 2 in the respiratory chain at the level of oxidative phosphorylation).
· REGULATION OF THE TCA CYCLE
The operating speed of the central heating system is precisely adjusted to needs cells in ATP, i.e. The Krebs cycle is associated with a respiratory chain that functions only under aerobic conditions. An important regulatory reaction of the cycle is the synthesis of citrate from acetyl-CoA and oxaloacetate, which occurs with the participation of citrate synthase. High ATP levels inhibit this enzyme. The second regulatory reaction of the cycle is isocitrate dehydrogenase. ADP and NAD + activate enzyme, NADH(H+) and ATP inhibit. The third regulatory reaction is oxidative decarboxylation of a-ketoglutarate. NADH(H+), succinyl-CoA and ATP inhibit a-ketoglutarate dehydrogenase.
· DISRUPTIONS OF THE CTK OPERATION
Violation The functioning of the central circulation system may be related to:
With a lack of acetyl-CoA;
With a lack of oxaloacetate (it is formed during the carboxylation of pyruvate, and the latter, in turn, during the breakdown of carbohydrates). An imbalance of the diet in carbohydrates entails the inclusion of acetyl-CoA in ketogenesis (the formation of ketone bodies), which leads to ketosis;
With a violation of the activity of enzymes due to a lack of vitamins that are part of the corresponding coenzymes (a lack of vitamin B 1 leads to a lack of TPP and disruption of the functioning of the a-ketoglutarate dehydrogenase complex; a lack of vitamin B 2 leads to a lack of FAD and a violation of the activity of succinate dehydrogenase; a lack of vitamin B 3 leads to is a deficiency of the coenzyme acylation CoA-SH and impaired activity of the a-ketoglutarate dehydrogenase complex; lack of vitamin B 5 leads to a lack of NAD and impaired activity of isocitrate dehydrogenase, a-ketoglutarate dehydrogenase complex and malate dehydrogenase; lack of lipoic acid also leads to impaired functioning of the a-ketoglutarate dehydrogenase complex);
With a lack of oxygen (hemoglobin synthesis and the functioning of the respiratory chain are impaired, and the accumulating NADH (H +) acts in this case as an allosteric inhibitor of isocitrate dehydrogenase and the a-ketoglutarate dehydrogenase complex)
· Control questions
I talked about what it actually is, why the Krebs cycle is needed and what place it occupies in metabolism. Now let's get down to the reactions of this cycle themselves.
I’ll make a reservation right away - for me personally, memorizing reactions was a completely pointless activity until I sorted out the above questions. But if you have already understood the theory, I suggest moving on to practice.
You can see many ways to write the Krebs cycle. The most common options are something like this:
But what seemed most convenient to me was the method of writing reactions from the good old textbook on biochemistry from the authors T.T. Berezov. and Korovkina B.V.
First reaction
The already familiar Acetyl-CoA and Oxaloacetate combine and turn into citrate, that is, into citric acid.

Second reaction
Now we take citric acid and turn it isocitric acid. Another name for this substance is isocitrate.
In fact, this reaction is somewhat more complicated, through an intermediate stage - the formation of cis-aconitic acid. But I decided to simplify it so that you remember it better. If necessary, you can add the missing step here if you remember everything else.
In essence, the two functional groups simply swapped places.

Third reaction
So, we have isocitric acid. Now it needs to be decarboxylated (that is, COOH is removed) and dehydrogenated (that is, H is removed). The resulting substance is a-ketoglutarate.
This reaction is notable for the formation of the HADH 2 complex. This means that the NAD transporter picks up hydrogen to start the respiratory chain.
I like the version of the Krebs Cycle reactions in the textbook by Berezov and Korovkin precisely because the atoms and functional groups that participate in the reactions are immediately clearly visible.

Fourth reaction
Again, nicotine Amide Adenine Dinucleotide works like clockwork, that is ABOVE. This nice carrier comes here, just like in the last step, to grab the hydrogen and carry it into the respiratory chain.
By the way, the resulting substance is succinyl-CoA, should not scare you. Succinate is another name for succinic acid, which is familiar to you from the days of bioorganic chemistry. Succinyl-Coa is a compound of succinic acid with coenzyme-A. We can say that this is an ester of succinic acid.

Fifth reaction
In the previous step, we said that succinyl-CoA is an ester of succinic acid. And now we will get the sama succinic acid, that is, succinate, from succinyl-CoA. An extremely important point: it is in this reaction that substrate phosphorylation.
Phosphorylation in general (it can be oxidative and substrate) is the addition of a phosphorus group PO 3 to GDP or ATP to obtain a complete GTF, or, respectively, ATP. The substrate differs in that this same phosphorus group is torn away from any substance containing it. Well, simply put, it is transferred from the SUBSTRATE to HDF or ADP. That’s why it’s called “substrate phosphorylation.”
Once again: at the beginning of substrate phosphorylation, we have a diphosphate molecule - guanosine diphosphate or adenosine diphosphate. Phosphorylation consists in the fact that a molecule with two phosphoric acid residues - HDP or ADP - is “completed” into a molecule with three phosphoric acid residues to produce guanosine TRIphosphate or adenosine TRIphosphate. This process occurs during the conversion of succinyl-CoA to succinate (i.e., succinic acid).
In the diagram you can see the letters F (n). It means "inorganic phosphate". Inorganic phosphate is transferred from the substrate to HDP so that the reaction products contain good, complete GTP. Now let's look at the reaction itself:

Sixth reaction
Next transformation. This time, the succinic acid that we obtained in the last step will turn into fumarate, note the new double bond.
The diagram clearly shows how it participates in the reaction FAD: This tireless carrier of protons and electrons picks up hydrogen and drags it directly into the respiratory chain.

Seventh reaction
We are already at the finish line. The penultimate stage of the Krebs Cycle is the reaction that converts fumarate to L-malate. L-malate is another name L-malic acid, familiar from the bioorganic chemistry course.
If you look at the reaction itself, you will see that, firstly, it goes both ways, and secondly, its essence is hydration. That is, fumarate simply attaches a water molecule to itself, resulting in L-malic acid.

Eighth reaction
The last reaction of the Krebs Cycle is the oxidation of L-malic acid to oxaloacetate, that is, to oxaloacetic acid. As you understand, “oxaloacetate” and “oxaloacetic acid” are synonyms. You probably remember that oxaloacetic acid is a component of the first reaction of the Krebs cycle.
Here we note the peculiarity of the reaction: formation of NADH 2, which will carry electrons into the respiratory chain. Don't forget also reactions 3,4 and 6, electron and proton carriers for the respiratory chain are also formed there.

As you can see, I specifically highlighted in red the reactions during which NADH and FADH2 are formed. These are very important substances for the respiratory chain. I highlighted in green the reaction in which substrate phosphorylation occurs and GTP is produced.
How to remember all this?
Actually, it's not that difficult. After reading my two articles in full, as well as your textbook and lectures, you just need to practice writing these reactions. I recommend remembering the Krebs cycle in blocks of 4 reactions. Write these 4 reactions several times, for each one choosing an association that suits your memory.
For example, I immediately very easily remembered the second reaction, in which isocitric acid is formed from citric acid (which, I think, is familiar to everyone from childhood).
You can also use mnemonics such as: " A Whole Pineapple and a Piece of Soufflé Is Actually My Lunch Today, which corresponds to the series - citrate, cis-aconitate, isocitrate, alpha-ketoglutarate, succinyl-CoA, succinate, fumarate, malate, oxaloacetate." There are a bunch more like them.
But, to be honest, I almost never liked such poems. In my opinion, it is easier to remember the sequence of reactions itself. It helped me a lot to divide the Krebs cycle into two parts, each of which I practiced writing several times an hour. As a rule, this happened in classes like psychology or bioethics. This is very convenient - without being distracted from the lecture, you can spend literally a minute writing the reactions as you remember them, and then check them with the correct option.
By the way, in some universities, during tests and exams in biochemistry, teachers do not require knowledge of the reactions themselves. You just need to know what the Krebs cycle is, where it occurs, what its features and significance are, and, of course, the chain of transformations itself. Only the chain can be named without formulas, using only the names of the substances. This approach is not without meaning, in my opinion.
I hope my guide to the TCA cycle has been helpful to you. And I want to remind you that these two articles are not a complete replacement for your lectures and textbooks. I wrote them only so that you roughly understand what the Krebs cycle is. If you suddenly see any error in my guide, please write about it in the comments. Thank you for your attention!
Brief historical information
Our favorite cycle is the TCA cycle, or the tricarboxylic acid cycle - life on Earth and under the Earth and in the Earth... Stop, in general this is the most amazing mechanism - it is universal, it is a way of oxidizing the breakdown products of carbohydrates, fats, proteins in the cells of living organisms, as a result We get energy for the activities of our body.
This process was discovered by Hans Krebs himself, for which he received the Nobel Prize!
He was born in August 25 - 1900 in the German city of Hildesheim. He received a medical education from the University of Hamburg and continued biochemical research under the leadership of Otto Warburg in Berlin.
In 1930, together with his student, he discovered the process of neutralizing ammonia in the body, which was present in many representatives of the living world, including humans. This cycle is the urea cycle, which is also known as the Krebs cycle #1.
When Hitler came to power, Hans emigrated to Great Britain, where he continues to study science at the Universities of Cambridge and Sheffield. Developing the research of the Hungarian biochemist Albert Szent-Györgyi, he received an insight and made the most famous Krebs cycle No. 2, or in other words, the “Szent-Györgyö – Krebs cycle” - 1937.
The research results are sent to the journal Nature, which refuses to publish the article. Then the text flies to the magazine "Enzymologia" in Holland. Krebs received the Nobel Prize in 1953 in physiology or medicine.
The discovery was surprising: in 1935 Szent-Györgyi found that succinic, oxaloacetic, fumaric and malic acids (all 4 acids are natural chemical components of animal cells) enhance the oxidation process in the pectoral muscle of the pigeon. Which was shredded.
It is in it that metabolic processes occur at the highest speed.
F. Knoop and K. Martius in 1937 found that citric acid is converted into isocitric acid through an intermediate product, cis - aconitic acid. In addition, isocitric acid could be converted into a-ketoglutaric acid, and that into succinic acid.
Krebs noticed the effect of acids on the absorption of O2 by the pectoral muscle of a pigeon and identified an activating effect on the oxidation of PVC and the formation of Acetyl-Coenzyme A. In addition, the processes in the muscle were inhibited by malonic acid, which is similar to succinic acid and could competitively inhibit enzymes whose substrate is succinic acid .
When Krebs added malonic acid to the reaction medium, the accumulation of a-ketoglutaric, citric and succinic acids began. Thus, it is clear that the combined action of a-ketoglutaric and citric acids leads to the formation of succinic acid.
Hans examined more than 20 other substances, but they did not affect oxidation. Comparing the data obtained, Krebs received a cycle. At the very beginning, the researcher could not say for sure whether the process began with citric or isocitric acid, so he called it the “tricarboxylic acid cycle.”
Now we know that the first is citric acid, so the correct name is the citrate cycle or the citric acid cycle.
In eukaryotes, TCA cycle reactions occur in mitochondria, while all enzymes for catalysis, except 1, are contained in a free state in the mitochondrial matrix; the exception is succinate dehydrogenase, which is localized on the inner membrane of the mitochondrion and is embedded in the lipid bilayer. In prokaryotes, the reactions of the cycle occur in the cytoplasm.
Let's meet the participants of the cycle:
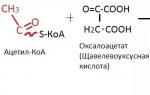
1) Acetyl Coenzyme A:- acetyl group
- coenzyme A - Coenzyme A:
2) PIKE – Oxaloacetate - Oxaloacetic acid:
seems to consist of two parts: oxalic and acetic acid.
3-4) Citric and Isocitric acids:
5) a-Ketoglutaric acid:
6) Succinyl-Coenzyme A:
7) Succinic acid:
8) Fumaric acid:
9) Malic acid:
How do reactions occur? In general, we are all accustomed to the appearance of the ring, which is shown below in the picture. Below everything is described step by step:
1. Condensation of Acetyl Coenzyme A and Oxaloacetic acid ➙ citric acid.
The transformation of Acetyl Coenzyme A begins with condensation with Oxaloacetic acid, resulting in the formation of citric acid.
The reaction does not require the consumption of ATP, since the energy for this process is provided as a result of hydrolysis of the thioether bond with Acetyl Coenzyme A, which is high-energy:
2. Citric acid passes through cis-aconitic acid into isocitric acid.
Isomerization of citric acid into isocitric acid occurs. The conversion enzyme - aconitase - first dehydrates citric acid to form cis-aconitic acid, then connects water to the double bond of the metabolite, forming isocitric acid:
3. Isocitric acid is dehydrogenated to form α-ketoglutaric acid and CO2.
Isocitric acid is oxidized by a specific dehydrogenase, the coenzyme of which is NAD.
Simultaneously with oxidation, decarboxylation of isocitric acid occurs. As a result of transformations, α-ketoglutaric acid is formed.
4. Alpha-ketoglutaric acid is dehydrogenated by ➙ succinyl-coenzyme A and CO2.
The next stage is the oxidative decarboxylation of α-ketoglutaric acid.
Catalyzed by the α-ketoglutarate dehydrogenase complex, which is similar in mechanism, structure and action to the pyruvate dehydrogenase complex. As a result, succinyl-CoA is formed.
5. Succinyl coenzyme A ➙ succinic acid.
Succinyl-CoA is hydrolyzed to free succinic acid, the energy released is stored by the formation of guanosine triphosphate. This stage is the only one in the cycle at which energy is directly released.
6. Succinic acid is dehydrogenated ➙ fumaric acid.
The dehydrogenation of succinic acid is accelerated by succinate dehydrogenase, its coenzyme is FAD.
7. Fumaric acid is hydrated ➙ malic acid.
Fumaric acid, which is formed by dehydrogenation of succinic acid, is hydrated and malic acid is formed.
8. Malic acid is dehydrogenated ➙ Oxalic-acetic acid - the cycle closes.
The final process is dehydrogenation of malic acid, catalyzed by malate dehydrogenase;
The result of the stage is the metabolite with which the tricarboxylic acid cycle begins - Oxalic-Acetic acid.
In reaction 1 of the next cycle, another quantity of Acetyl Coenzyme A will enter.
How to remember this cycle? Just!
1) A very figurative expression:A Whole Pineapple and a Piece of Soufflé Is Actually My Lunch Today, which corresponds to - citrate, cis-aconitate, isocitrate, (alpha-)ketoglutarate, succinyl-CoA, succinate, fumarate, malate, oxaloacetate.
2) Another long poem:
PIKE ate acetate, it turns out citrate,
Through cisaconitate it will become isocitrate.
Having given up hydrogen to NAD, it loses CO2,
Alpha-ketoglutarate is extremely happy about this.
Oxidation is coming - NAD has stolen hydrogen,
TDP, coenzyme A takes CO2.
And the energy barely appeared in succinyl,
Immediately ATP was born and what remained was succinate.
Now he got to the FAD - he needs hydrogen,
The fumarate drank from the water and turned into malate.
Then NAD came to malate, acquired hydrogen,
The PIKE showed up again and quietly hid.
3) The original poem - in short:
PIKE ACETYL LIMONIL,
But the horse was afraid of narcissus,
He is above him ISOLIMON
ALPHA - KETOGLUTARASED.
SUCCINALIZED WITH COENZYME,
AMBER FUMAROVO,
Stored up some APPLES for the winter,
Turned into a PIKE again.
TRICARBOXYLIC ACIDS CYCLE (KREBS CYCLE)
Glycolysis converts glucose into pyruvate and produces two ATP molecules from a glucose molecule—a small fraction of that molecule's potential energy.
Under aerobic conditions, pyruvate is converted from glycolysis to acetyl-CoA and oxidized to CO2 in the tricarboxylic acid cycle (citric acid cycle). In this case, the electrons released in the reactions of this cycle pass through NADH and FADH 2 to 0 2 - the final acceptor. Electron transport is associated with the creation of a proton gradient in the mitochondrial membrane, the energy of which is then used for the synthesis of ATP as a result of oxidative phosphorylation. Let's consider these reactions.
Under aerobic conditions, pyruvic acid (1st stage) undergoes oxidative decarboxylation, more efficient than transformation into lactic acid, with the formation of acetyl-CoA (2nd stage), which can be oxidized to the final products of glucose breakdown - CO 2 and H 2 0 (3rd stage). G. Krebs (1900-1981), a German biochemist, having studied the oxidation of individual organic acids, combined their reactions into a single cycle. Therefore, the tricarboxylic acid cycle is often called the Krebs cycle in his honor.
The oxidation of pyruvic acid to acetyl-CoA occurs in mitochondria with the participation of three enzymes (pyruvate dehydrogenase, lipoamide dehydrogenase, lipoyl acetyltransferase) and five coenzymes (NAD, FAD, thiamine pyrophosphate, lipoic acid amide, coenzyme A). These four coenzymes contain B vitamins (B x, B 2, B 3, B 5), which indicates the need for these vitamins for the normal oxidation of carbohydrates. Under the influence of this complex enzyme system, pyruvate is converted in an oxidative decarboxylation reaction into the active form of acetic acid - acetyl coenzyme A:
Under physiological conditions, pyruvate dehydrogenase is an exclusively irreversible enzyme, which explains the impossibility of converting fatty acids into carbohydrates.
The presence of a high-energy bond in the acetyl-CoA molecule indicates the high reactivity of this compound. In particular, acetyl-CoA can act in mitochondria to generate energy; in the liver, excess acetyl-CoA is used for the synthesis of ketone bodies; in the cytosol it participates in the synthesis of complex molecules such as steroids and fatty acids.
Acetyl-CoA obtained in the reaction of oxidative decarboxylation of pyruvic acid enters the tricarboxylic acid cycle (Krebs cycle). The Krebs cycle, the final catabolic pathway for the oxidation of carbohydrates, fats, and amino acids, is essentially a “metabolic cauldron.” The reactions of the Krebs cycle, which occur exclusively in mitochondria, are also called the citric acid cycle or the tricarboxylic acid cycle (TCA cycle).
One of the most important functions of the tricarboxylic acid cycle is the generation of reduced coenzymes (3 molecules of NADH + H + and 1 molecule of FADH 2) followed by the transfer of hydrogen atoms or their electrons to the final acceptor - molecular oxygen. This transport is accompanied by a large decrease in free energy, part of which is used in the process of oxidative phosphorylation for storage in the form of ATP. It is clear that the tricarboxylic acid cycle is aerobic, oxygen dependent.
1. The initial reaction of the tricarboxylic acid cycle is the condensation of acetyl-CoA and oxaloacetic acid with the participation of the mitochondrial matrix enzyme citrate synthase to form citric acid.

2. Under the influence of the enzyme aconitase, which catalyzes the removal of a water molecule from citrate, the latter turns

to cis-aconitic acid. Water combines with cis-aconitic acid, turning into isocitric acid.
3. The enzyme isocitrate dehydrogenase then catalyzes the first dehydrogenase reaction of the citric acid cycle, when isocitric acid is converted by oxidative decarboxylation to α-ketoglutaric acid:

In this reaction, the first molecule of CO 2 and the first molecule of NADH 4- H + cycle are formed.
4. Further conversion of α-ketoglutaric acid to succinyl-CoA is catalyzed by the multienzyme complex of α-ketoglutaric dehydrogenase. This reaction is chemically analogous to the pyruvate dehydrogenase reaction. It involves lipoic acid, thiamine pyrophosphate, HS-KoA, NAD +, FAD. 
As a result of this reaction, a NADH + H + and CO 2 molecule is again formed.
5. The succinyl-CoA molecule has a high-energy bond, the energy of which is stored in the next reaction in the form of GTP. Under the influence of the enzyme succinyl-CoA synthetase, succinyl-CoA is converted into free succinic acid. Note that succinic acid can also be obtained from methylmalonyl-CoA by oxidation of fatty acids with an odd number of carbon atoms.

This reaction is an example of substrate phosphorylation, since the high-energy GTP molecule in this case is formed without the participation of the electron and oxygen transport chain.
6. Succinic acid is oxidized to fumaric acid in the succinate dehydrogenase reaction. Succinate dehydrogenase, a typical iron-sulfur-containing enzyme, the coenzyme of which is FAD. Succinate dehydrogenase is the only enzyme anchored to the inner mitochondrial membrane, while all other cycle enzymes are located in the mitochondrial matrix. 
7. This is followed by the hydration of fumaric acid into malic acid under the influence of the enzyme fumarase in a reversible reaction under physiological conditions:

8. The final reaction of the tricarboxylic acid cycle is the malate dehydrogenase reaction with the participation of the active enzyme mitochondrial NAD~-dependent malate dehydrogenase, in which the third molecule of reduced NADH + H + is formed:

The formation of oxaloacetic acid (oxaloacetate) completes one revolution of the tricarboxylic acid cycle. Oxalacetic acid can be used in the oxidation of a second molecule of acetyl-CoA, and this cycle of reactions can be repeated many times, constantly leading to the production of oxaloacetic acid.
Thus, the oxidation of one molecule of acetyl-CoA in the TCA cycle as a substrate of the cycle leads to the production of one molecule of GTP, three molecules of NADP + H + and one molecule of FADH 2. Oxidation of these reducing agents in the biological oxidation chain

lenition leads to the synthesis of 12 ATP molecules. This calculation is clear from the topic “Biological oxidation”: the inclusion of one NAD + molecule in the electron transport system is ultimately accompanied by the formation of 3 ATP molecules, the inclusion of a FADH 2 molecule ensures the formation of 2 ATP molecules, and one GTP molecule is equivalent to 1 ATP molecule.
Note that two carbon atoms of adetyl-CoA enter the tricarboxylic acid cycle and two carbon atoms leave the cycle as CO 2 in decarboxylation reactions catalyzed by isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase.
With the complete oxidation of a glucose molecule under aerobic conditions to C0 2 and H 2 0, the formation of energy in the form of ATP is:
- 4 molecules of ATP during the conversion of a glucose molecule into 2 molecules of pyruvic acid (glycolysis);
- 6 ATP molecules formed in the 3-phosphoglyceraldehyde dehydrogenase reaction (glycolysis);
- 30 ATP molecules formed during the oxidation of two molecules of pyruvic acid in the pyruvate dehydrogenase reaction and in the subsequent transformations of two molecules of acetyl-CoA to CO 2 and H 2 0 in the tricarboxylic acid cycle. Therefore, the total energy output from complete oxidation of a glucose molecule can be 40 ATP molecules. However, it should be taken into account that during the oxidation of glucose, two ATP molecules are consumed at the stage of converting glucose into glucose-6-phosphate and at the stage of converting fructose-6-phosphate into fructose-1,6-diphosphate. Therefore, the “net” energy output from the oxidation of a glucose molecule is 38 ATP molecules.
You can compare the energetics of anaerobic glycolysis and aerobic catabolism of glucose. Of the 688 kcal of energy theoretically contained in 1 gram molecule of glucose (180 g), 20 kcal is in two molecules of ATP formed in the reactions of anaerobic glycolysis, and 628 kcal theoretically remains in the form of lactic acid.
Under aerobic conditions, from 688 kcal of a gram molecule of glucose in 38 ATP molecules, 380 kcal are obtained. Thus, the efficiency of glucose use under aerobic conditions is approximately 19 times higher than in anaerobic glycolysis.
It should be noted that all oxidation reactions (oxidation of triose phosphate, pyruvic acid, four oxidation reactions of the tricarboxylic acid cycle) compete in the synthesis of ATP from ADP and phosphorus (Pasteur effect). This means that the resulting molecule NADH + H + in oxidation reactions has a choice between the reactions of the respiratory system, transferring hydrogen to oxygen, and the enzyme LDH, transferring hydrogen to pyruvic acid.
In the early stages of the tricarboxylic acid cycle, its acids can leave the cycle to participate in the synthesis of other cell compounds without disrupting the functioning of the cycle itself. Various factors are involved in the regulation of tricarboxylic acid cycle activity. Among them, primarily the supply of acetyl-CoA molecules, the activity of the pyruvate dehydrogenase complex, the activity of the components of the respiratory chain and associated oxidative phosphorylation, as well as the level of oxaloacetic acid should be mentioned.
Molecular oxygen is not directly involved in the tricarboxylic acid cycle, but its reactions are carried out only under aerobic conditions, since NAD ~ and FAD can be regenerated in mitochondria only by transferring electrons to molecular oxygen. It should be emphasized that glycolysis, in contrast to the tricarboxylic acid cycle, is also possible under anaerobic conditions, since NAD~ is regenerated during the transition of pyruvic acid to lactic acid.
In addition to the formation of ATP, the tricarboxylic acid cycle has another important meaning: the cycle provides intermediary structures for various biosyntheses of the body. For example, most of the atoms of porphyrins come from succinyl-CoA, many amino acids are derivatives of α-ketoglutaric and oxaloacetic acids, and fumaric acid occurs in the process of urea synthesis. This demonstrates the integrity of the tricarboxylic acid cycle in the metabolism of carbohydrates, fats, and proteins.
As the reactions of glycolysis show, the ability of most cells to generate energy lies in their mitochondria. The number of mitochondria in various tissues is associated with the physiological functions of the tissues and reflects their ability to participate in aerobic conditions. For example, red blood cells do not have mitochondria and therefore do not have the ability to generate energy using oxygen as the final electron acceptor. However, in cardiac muscle functioning under aerobic conditions, half the volume of the cell cytoplasm is represented by mitochondria. The liver also depends on aerobic conditions for its various functions, and mammalian hepatocytes contain up to 2 thousand mitochondria per cell.
Mitochondria include two membranes - outer and inner. The outer membrane is simpler, consisting of 50% fats and 50% proteins, and has relatively few functions. The inner membrane is structurally and functionally more complex. Approximately 80% of its volume is proteins. It contains most of the enzymes involved in electron transport and oxidative phosphorylation, metabolic intermediaries and adenine nucleotides between the cytosol and the mitochondrial matrix.
Various nucleotides involved in redox reactions, such as NAD +, NADH, NADP +, FAD and FADH 2, do not penetrate the inner mitochondrial membrane. Acetyl-CoA cannot move from the mitochondrial compartment to the cytosol, where it is required for the synthesis of fatty acids or sterols. Therefore, intramitochondrial acetyl-CoA is converted into the citrate synthase reaction of the tricarboxylic acid cycle and enters the cytosol in this form.
The tricarboxylic acid cycle was first discovered by the English biochemist Krebs. He was the first to postulate the importance of this cycle for the complete combustion of pyruvate, the main source of which is the glycolytic conversion of carbohydrates. It was subsequently shown that the tricarboxylic acid cycle is a “focus” at which almost all metabolic pathways converge.
So, acetyl-CoA formed as a result of oxidative decarboxylation of pyruvate enters the Krebs cycle. This cycle consists of eight consecutive reactions (Fig. 91). The cycle begins with the condensation of acetyl-CoA with oxaloacetate and the formation of citric acid. ( As will be seen below, in the cycle it is not acetyl-CoA itself that undergoes oxidation, but a more complex compound - citric acid (tricarboxylic acid).)
Then citric acid (a six-carbon compound), through a series of dehydrogenations (removal of hydrogen) and decarboxylation (elimination of CO 2), loses two carbon atoms and again oxaloacetate (a four-carbon compound) appears in the Krebs cycle, i.e., as a result of a complete revolution of the cycle, the acetyl-CoA molecule burns to CO 2 and H 2 O, and the oxaloacetate molecule is regenerated. Below are all eight sequential reactions (stages) of the Krebs cycle.
In the first reaction, catalyzed by the enzyme citrate synthase, acetyl-CoA is condensed with oxaloacetate. As a result, citric acid is formed:

Apparently, in this reaction, citril-CoA bound to the enzyme is formed as an intermediate product. The latter is then spontaneously and irreversibly hydrolyzed to form citrate and HS-CoA.
In the second reaction of the cycle, the resulting citric acid undergoes dehydration to form cis-aconitic acid, which, by adding a water molecule, becomes isocitric acid. These reversible hydration-dehydration reactions are catalyzed by the enzyme aconitate hydratase:

In the third reaction, which appears to be the rate-limiting reaction of the Krebs cycle, isocitric acid is dehydrogenated in the presence of NAD-dependent isocitrate dehydrogenase:

(There are two types of isocitrate dehydrogenases in tissues: NAD- and NADP-dependent. It has been established that NAD-dependent isocitrate dehydrogenase plays the role of the main catalyst for the oxidation of isocitric acid in the Krebs cycle.)
During the isocitrate dehydrogenase reaction, isocitric acid is decarboxylated. NAD-dependent isocitrate dehydrogenase is an allosteric enzyme that requires ADP as a specific activator. In addition, the enzyme requires Mg 2+ or Mn 2+ ions to exhibit its activity.
In the fourth reaction, α-ketoglutaric acid is oxidatively decarboxylated to succinyl-CoA. The mechanism of this reaction is similar to the reaction of oxidative decarboxylation of pyruvate to acetyl-CoA. The α-ketoglutarate dehydrogenase complex is similar in structure to the pyruvate dehydrogenase complex. In both cases, five coenzymes take part in the reaction: TDP, lipoic acid amide, HS-CoA, FAD and NAD. In total, this reaction can be written as follows:

The fifth reaction is catalyzed by the enzyme succinyl-CoA synthetase. During this reaction, succinyl-CoA, with the participation of GDP and inorganic phosphate, is converted into succinic acid (succinate). At the same time, the formation of a high-energy phosphate bond of GTP1 occurs due to the high-energy thioester bond of succinyl-CoA:

(The resulting GTP then donates its terminal phosphate group to ADP, resulting in the formation of ATP. The formation of a high-energy nucleoside triphosphate during the succinyl-CoA synthetase reaction is an example of phosphorylation at the substrate level.)
In the sixth reaction, succinate is dehydrogenated to fumaric acid. The oxidation of succinate is catalyzed by succinate dehydrogenase, in the molecule of which the coenzyme FAD is covalently bound to the protein:

In the seventh reaction, the resulting fumaric acid is hydrated under the influence of the enzyme fumarate hydratase. The product of this reaction is malic acid (malate). It should be noted that fumarate hydratase is stereospecific - during this reaction L-malic acid is formed:

Finally, in the eighth reaction of the tricarboxylic acid cycle, under the influence of mitochondrial NAD-dependent malate dehydrogenase, L-malate is oxidized to oxaloacetate:

As you can see, in one turn of the cycle, consisting of eight enzymatic reactions, complete oxidation (“combustion”) of one molecule of acetyl-CoA occurs. For continuous operation of the cycle, a constant supply of acetyl-CoA into the system is necessary, and coenzymes (NAD and FAD), which have passed into a reduced state, must be oxidized again and again. This oxidation occurs in the electron transport system (or chain of respiratory enzymes) located in the mitochondria.
The energy released as a result of the oxidation of acetyl-CoA is largely concentrated in the high-energy phosphate bonds of ATP. Of the four pairs of hydrogen atoms, three pairs are transferred through NAD to the electron transport system; in this case, for each pair in the biological oxidation system, three ATP molecules are formed (in the process of conjugate oxidative phosphorylation), and therefore a total of nine ATP molecules. One pair of atoms enters the electron transport system through FAD, resulting in the formation of 2 ATP molecules. During the reactions of the Krebs cycle, 1 molecule of GTP is also synthesized, which is equivalent to 1 molecule of ATP. So, the oxidation of acetyl-CoA in the Krebs cycle produces 12 ATP molecules.

As already noted, 1 molecule of NADH 2 (3 molecules of ATP) is formed during the oxidative decarboxylation of pyruvate into acetyl-CoA. Since the breakdown of one molecule of glucose produces two molecules of pyruvate, when they are oxidized to 2 molecules of acetyl-CoA and the subsequent two turns of the tricarboxylic acid cycle, 30 molecules of ATP are synthesized (hence, the oxidation of one molecule of pyruvate to CO 2 and H 2 O produces 15 molecules ATP).
To this we must add 2 ATP molecules formed during aerobic glycolysis, and 4 ATP molecules synthesized through the oxidation of 2 molecules of extramitochondrial NADH 2, which are formed during the oxidation of 2 molecules of glyceraldehyde-3-phosphate in the dehydrogenase reaction. In total, we find that when 1 glucose molecule is broken down in tissues according to the equation: C 6 H 12 0 6 + 60 2 -> 6CO 2 + 6H 2 O, 36 ATP molecules are synthesized, which contributes to the accumulation of adenosine triphosphate in high-energy phosphate bonds 36 X 34.5 ~ 1240 kJ (or, according to other sources, 36 X 38 ~ 1430 kJ) free energy. In other words, of all the free energy released during aerobic oxidation of glucose (about 2840 kJ), up to 50% of it is accumulated in mitochondria in a form that can be used to perform various physiological functions. There is no doubt that, energetically, the complete breakdown of glucose is a more efficient process than glycolysis. It should be noted that the NADH 2 molecules formed during the conversion of glyceraldehyde-3-phosphate 2 subsequently, upon oxidation, produce not 6 ATP molecules, but only 4. The fact is that the molecules of extramitochondrial NADH 2 themselves are not able to penetrate through the membrane into the mitochondria. However, the electrons they donate can be included in the mitochondrial chain of biological oxidation using the so-called glycerophosphate shuttle mechanism (Fig. 92). As can be seen in the figure, cytoplasmic NADH 2 first reacts with cytoplasmic dihydroxyacetone phosphate to form glycerol 3-phosphate. The reaction is catalyzed by NAD-dependent cytoplasmic glycerol-3-phosphate dehydrogenase.